Scientists at MIT created a nanoparticle, that could boost the capacity and power of lithium-ion batteries.
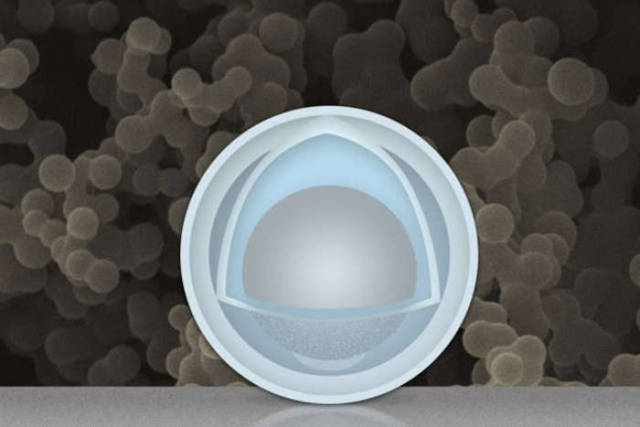
The gray sphere at center represents an aluminum nanoparticle, forming the “yolk.” The outer light-blue layer represents a solid shell of titanium dioxide, and the space in between the yolk and shell allows the yolk to expand and contract without damaging the shell. In the background is an actual scanning electron microscope image of a collection of these yolk-shell nanoparticles. Credit: Christine Daniloff/MIT
A team of researchers at MIT and Tsinghua University in China has found a novel way around the problem faced by electrodes in rechargeable batteries, that increase and decrease in volume, as they go through repeated cycles of charging and discharging.
Aluminum could give a big boost to capacity and power of lithium-ion batteries.
They created an electrode made of nanoparticles with a solid shell, and a “yolk” inside that can change size again and again without affecting the shell. The innovation could drastically improve cycle life, the team says, and provide a dramatic boost in the battery’s capacity and power.
The new findings, which use aluminum as the key material for the lithium-ion battery’s negative electrode, or anode, are reported in the journal Nature Communications, in a paper by MIT professor Ju Li and six others. The use of nanoparticles with an aluminum yolk and a titanium dioxide shell has proven to be “the high-rate champion among high-capacity anodes,” the team reports.
source MIT



Leave A Comment