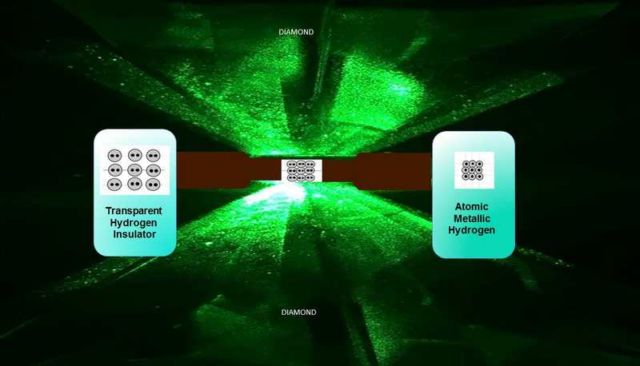
Using two diamonds, scientists squeezed hydrogen, to pressures above those in Earth’s core, to create metallic hydrogen.
Nearly a century after it was theorized, Harvard scientists have succeeded in creating metallic hydrogen.
Physicists by applying 4.95 million atmospheres of pressure to liquid hydrogen, succeed to make it metallic.
This could revolutionize everything from energy storage to rocketry.
Above, image of a diamond anvil cell compressing molecular hydrogen. At 4.95 million atmospheres, the sample converts to metallic hydrogen, as shown on the right. Credit R. Dias and I.F. Silvera
In addition to helping scientists answer fundamental questions about the nature of matter, the material is theorized to have a wide range of applications, ranging from room-temperature superconductors to powerful rocket propellant.
Isaac Silvera, professor of the natural sciences, described the accomplishment in a recent Harvard press release:
“This is the Holy Grail of high-pressure physics. It’s the first-ever sample of metallic hydrogen on Earth, so when you’re looking at it, you’re looking at something that’s never existed before.”
“One prediction that’s very important is metallic hydrogen is predicted to be meta-stable. That means if you take the pressure off, it will stay metallic, similar to the way diamonds form from graphite under intense heat and pressure, but remain diamonds when that pressure and heat are removed. As much as 15 percent of energy is lost to dissipation during transmission, so if you could make wires from this material and use them in the electrical grid, it could change that story.”
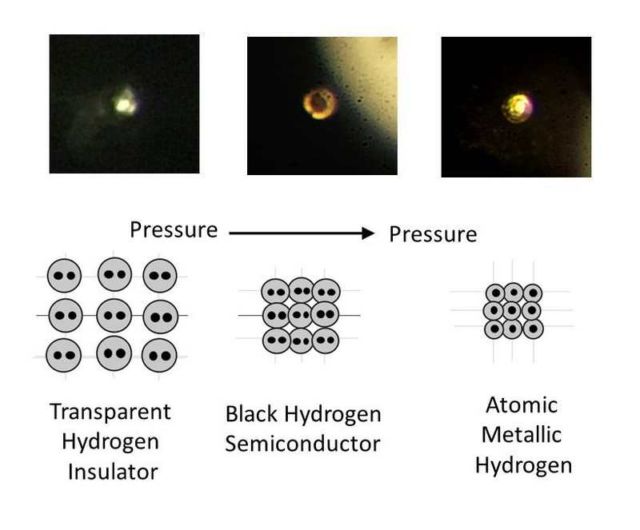
Images of compressed hydrogen transitioning with increasing pressure from transparent molecular to black molecular to atomic metallic hydrogen. Credit R. Dias and I.F. Silvera
via universetoday
sources harvard, harvardmagazine





Leave A Comment