Scientists freeze molecule to 500 nanokelvins, a temp that’s nearly absolute zero.
A team of MIT scientists managed to freeze a molecule, by successfully cooling a gas of sodium potassium (NaK) molecules to a temperature of 500 nanokelvin.
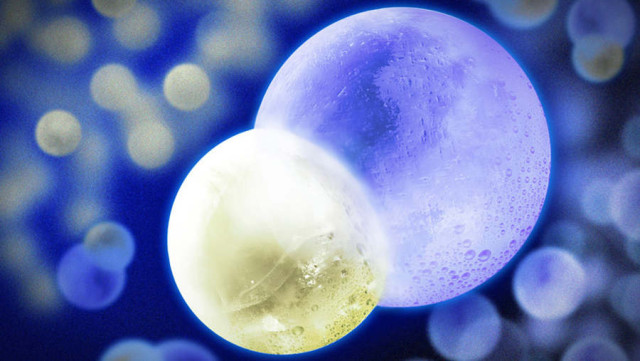
Above image: In this artist’s illustration, the NaK molecule is represented with frozen spheres of ice merged together: the smaller sphere on the left represents a sodium atom, and the larger sphere on the right is a potassium atom. Illustration: Jose-Luis Olivares/MIT
The absolute zero (0 Kelvin) is around -459.67 degrees F, or −273.15 degrees Celsius.
According to the researchers in near absolute zero, molecules may start to exhibit ‘exotic states of matter.’
Scientists have long suspected that if temperatures were to plunge to near absolute zero, molecules would come to a screeching halt, ceasing their individual chaotic motion and behaving as one collective body. This more orderly molecular behavior would begin to form very strange, exotic states of matter — states that have never been observed in the physical world.
Now experimental physicists at MIT have successfully cooled molecules in a gas of sodium potassium (NaK) to a temperature of 500 nanokelvins — just a hair above absolute zero, and over a million times colder than interstellar space. The researchers found that the ultracold molecules were relatively long-lived and stable, resisting reactive collisions with other molecules. The molecules also exhibited very strong dipole moments — strong imbalances in electric charge within molecules that mediate magnet-like forces between molecules over large distances.
Martin Zwierlein, professor of physics at MIT and a principal investigator in MIT’s Research Laboratory of Electronics, says that ‘while molecules are normally full of energy, vibrating and rotating and moving through space at a frenetic pace, the group’s ultracold molecules have been effectively stilled — cooled to average speeds of centimeters per second and prepared in their absolute lowest vibrational and rotational states.’
“We are very close to the temperature at which quantum mechanics plays a big role in the motion of molecules. So these molecules would no longer run around like billiard balls, but move as quantum mechanical matter waves. And with ultracold molecules, you can get a huge variety of different states of matter, like superfluid crystals, which are crystalline, yet feel no friction, which is totally bizarre. This has not been observed so far, but predicted. We might not be far from seeing these effects, so we’re all excited.”
source MIT




Leave A Comment